The story of 1-butanesulfonic acid traces back to deeper studies on organic sulfonic compounds. Chemists sought strong acids capable of acting as catalysts and intermediates, but less harsh than mineral acids. Progress in sulfonation chemistry in the late 1800s and early 1900s led to several aliphatic sulfonic acids, and 1-butanesulfonic acid attracted attention due to its manageable carbon chain and convenient melting range. Over the decades, tweaks in synthetic processes, safer handling techniques, and better understanding of structure-activity relationships brought it out from a chemical oddity into an important industrial tool.
1-Butanesulfonic acid generally appears as a colorless to slightly yellow liquid or low-melting solid. Its unmistakable acidic odor signals caution during use. Commercial offerings come in varying grades including technical, reagent, and occasionally pharmaceutical grades. Production scales reach from laboratory catalysts to bulk shipments destined for large-scale synthesis. Companies across Europe, Asia, and North America supply it, underscoring its wide acceptance in chemical reactivity schemes, especially where aqueous processes require strong acids that dissolve well in both water and organic solvents.
With a molecular formula of C4H10O3S and a molar mass of 138.19 g/mol, 1-butanesulfonic acid blends compactness with functional punch. Boiling points hover near 245°C, while melting points can drop below 20°C, reflecting its relatively low molecular weight and the bulky sulfonic group. Deliquescence and hygroscopicity show up in humid environments, so containers demand sealing. The acid freely dissolves in water, methanol, and ethanol, though solubility in pure hydrocarbons remains limited. Strong acidity characterizes the molecule due to the sulfonic function, often matching or exceeding other straight-chain alkanesulfonic acids. I’ve worked with similar small-chain sulfonic acids in the lab before, and the faint sulfurous note always served as a warning to limit inhalation.
Producers assign purity ratings according to industry needs—most lots exceed 98% by titration or HPLC analysis, and water content sits below 1%. Impurity profiles might detail residual starting material, sulfur dioxide, or traces of unreacted alkanes. Each container requires detailed labeling with identifiers, recommended storage conditions, lot number for traceability, and relevant hazard information, including GHS pictograms for irritation and environmental hazard. Even for smaller packages shipped to research labs, safety datasheets outline all possible risks and required personal protection, based on both regulatory standards and prior incident records.
Its preparation proceeds through the sulfonation of n-butane or 1-butanol. Interest in direct sulfonation arose because of the environmental concerns about leftover halides from older synthetic paths. The liquid phase oxidation of n-butane first forms 1-butanesulfonic acid salts, which undergo acidification with strong mineral acids. Another option starts with 1-butanol, which gets treated with concentrated sulfuric acid under controlled heating. Each step brings handling risks—corrosive behavior, exothermic reactions, gas release—so process control technology must track temperature, gas evolution, and pH shifts. Scaling from gram-level syntheses to ton-scale production needs closed systems and rigorous venting to keep workers safe and product uncontaminated.
High reactivity marks the sulfonic acid function: transformations range from esterification to salt formation, making it a go-to intermediate for custom surfactants and ionic liquids. Neutralization with alkali produces butanesulfonates, which act as counterions for drug formulations and catalysts for phase-transfer reactions. The free acid also pushes forward substitution reactions on activated aromatic rings, and chemists have modified it into tens of bespoke derivatives with altered solubility or reactivity. On large-scale setups, coupling 1-butanesulfonic acid with organic bases builds on-demand proton sources for catalytic hydrogenation and hydrolysis processes. It doesn’t just stick to one niche; versatility and predictability in its sulfur chemistry allow wide-ranging applications.
Clarity in naming ensures users don’t confuse this compound with other isomeric or chain-length relatives. Besides “1-butanesulfonic acid,” you’ll see “n-butanesulfonic acid,” “butylsulfonic acid,” and sometimes “butanesulphonic acid” as per British spelling conventions. In catalogs, it may appear under its CAS number for quick reference or as “BSFA” in process notes and technical papers. Regularly, knowing alternate names matters: confusing it with 2-butanesulfonic acid or isobutanesulfonic acid can cause misdirected or failed syntheses and disrupt supply-chain reliability.
Strict chemical hygiene governs all work with 1-butanesulfonic acid. Direct contact causes skin and respiratory irritation, while chronic exposure might harm mucous membranes. Protective gloves, goggles, and chemical-resistant clothing make up standard practice; I’ve learned the hard way that casual handling, even for brief pipetting, leads to some nasty rashes. Local exhaust ventilation controls vapors, particularly when handling the heated liquid. Storage in cool, dry, well-ventilated spaces, away from strong oxidizers and incompatible bases, heads off fires and unwanted reactions. Emergency procedures cover neutralization with sodium bicarbonate and rapid evacuation in the event of a spill. Documentation stands at the heart of safe use: up-to-date safety datasheets, clear signage, and routine safety drills minimize human error and occupational risk.
1-Butanesulfonic acid features in a surprising spectrum of industrial and lab uses. It enters the manufacture of specialty surfactants that lower surface tension in cleaning products, emulsifiers for agrochemicals, and even particles for the electronics industry where material purity sits above all else. In pharmaceutical analysis, its high solubility and ionic character serve as a mobile phase additive for HPLC, stabilizing tricky compounds during separation. Electroplating and battery chemistry both benefit from sulfonic acid’s properties, where standard mineral acids fail by promoting unwanted side-reactions or poor layer formation. I’ve seen demand rise in green chemistry applications, replacing older, less sustainable acids in catalysts and solvents recycling streams.
R&D teams stay busy pushing the boundaries of sulfonic acid chemistry, searching for greener syntheses and novel reactivity. Modifying the butane skeleton aims to fine-tune acidity or compatibility with polymers, shifting focus from bulk commodity to tailored specialty chemicals. Some researchers dive into the environmental fate of 1-butanesulfonic acid, tracing breakdown products in wastewater from detergent plants or battery cell manufacturing. Each round of publications brings new insight into reactivity mechanisms, stabilization strategies, and ways to reduce corrosion or toxicity in applied settings. Universities and industrial labs both take up pilot projects where this compound acts as a benchmark, often serving as the standard of comparison for up-and-coming organic acids.
Data from toxicity studies forms the basis for all regulatory controls. Acute exposure to concentrated vapor or liquid causes respiratory distress, eye irritation, and sometimes burns; extended contact raises risks of dermatitis or, for sensitive individuals, allergic sensitization. Animal studies highlight low oral LD50 values, suggesting moderate oral toxicity in comparison to other sulfonic acids. No evidence suggests significant carcinogenicity, but the thorough breakdown of metabolic pathways remains a work in progress, especially given the lack of long-term epidemiological studies in industrial settings. Waste streams from manufacture must undergo monitored neutralization, as untreated release can provoke aquatic toxicity—something I’ve witnessed during analytical testing campaigns in environmental monitoring.
The future demands more from 1-butanesulfonic acid. The rise of sustainable chemistry, renewable feedstocks, and non-toxic alternatives keeps pushing producers to refine manufacturing methods. Remote monitoring of key quality attributes, automation, and less hazardous reagents point the way toward improved worker safety and lower environmental impact. Analysts also forecast strong demand from lithium-ion battery manufacturers, water treatment specialists, and pharmaceutical process designers. I’ve seen small changes—whether in synthetic routes or purification techniques—unlock new areas like biopolymer modification and ionic liquid engineering. Real progress comes from keeping safety and environmental responsibility in focus, without forgetting the reliable performance that built 1-butanesulfonic acid’s solid reputation.
1-Butanesulfonic acid pops up in labs across the world. To a lot of people, this chemical isn’t something you see on store shelves, but it quietly influences many products we use every day. It’s an organosulfonic acid made of four carbon atoms along a chain, ending with a sulfonic acid group. This simple structure hides its surprisingly broad impact.
In the world of chemistry, separating things cleanly is gold. Scientists rely on high-performance liquid chromatography (HPLC) to tell molecules apart with great precision. 1-Butanesulfonic acid helps in separating and identifying molecules that would otherwise stick together or blend into the background. This is especially true for charged molecules like pharmaceutical compounds or peptides. The acid works as an ion-pairing agent, which means it latches onto molecules, giving them just the right amount of charge to help them drift apart at a controlled pace. This little boost improves accuracy, boosts reliability, and helps researchers spot details that would get lost.
Factories that produce dyes, pigments, and detergents pick up 1-butanesulfonic acid when they want to push reactions in a certain direction. Its sulfonic acid group plays a huge role in catalyzing reactions. My own colleagues tell stories of how a batch of specialty colorant only worked right when they switched to this acid as the catalyst. By giving reactions more consistency, companies waste less, turning out purer products that meet strict standards. The chemical world often struggles with controlling side-reactions, and this acid, because of its unique blend of stability and reactivity, helps cut down on unwanted byproducts.
No one talks much about the background chemistry holding tablets and injections together, but it matters. Medicine has to be stable—nobody wants degraded drugs. In certain active pharmaceutical ingredients (APIs), 1-butanesulfonic acid steps up as a salt-forming agent. This helps the medicine dissolve well and hang onto its punch during storage. It may help in reducing bitterness or improving shelf life. I’ve read case studies where a drug that flopped in early tests bounced back just through reformulation with the right acid to form a salt.
This chemical doesn’t come without its headaches. Handling acids takes training and strict safety rules. Some countries demand extra paperwork and labeling. Besides worker safety, industry leans toward greener practices. Large scale chemical use always means keeping an eye on runoff and breakdown products. Some research dives into biodegradable alternatives, or tweaks the process to capture and recycle spent chemicals, including this one. There’s momentum behind developing purification systems that catch and neutralize chemical waste before it escapes powdered walls and fume hoods.
Lab chemists and industrial managers face a balancing act. Cutting costs shouldn’t outweigh thinking about long-term effects. Sharing best practices matters just as much as new lab discoveries. From what I’ve seen, companies succeed when they invest in good training, keep honest records, and switch toward less hazardous methods where possible. Growing demand for safe and transparent manufacture keeps 1-butanesulfonic acid in the spotlight for anyone crafting policies or products.
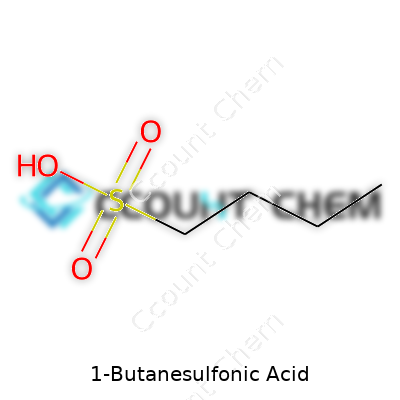
1-Butanesulfonic acid carries the formula C4H10O3S. On paper, these numbers and letters might not grab attention. In a chemistry lab, though, this string of symbols represents a molecule that sticks together with a backbone of four carbons—the butane part—with a sulfonic acid group holding on at one end. That functional group isn’t just window dressing; it gives the compound its strong acidic nature, letting it dive straight into reactions that many other organic molecules shy away from.
Over the years, I’ve seen a lot of molecules come and go, each one with its place on the periodic table of usefulness. 1-Butanesulfonic acid stands out because of how easily that sulfonic acid group latches onto things. Chemists in both research and industry have leaned on it for a long time, whether they’re aiming to drive a particular reaction or split up tricky mixtures. The compound earns its keep in ion chromatography, where separating and identifying smaller ions could turn into a guessing game without the right reagents. When you bring 1-Butanesulfonic acid into the mix, it acts like a traffic controller, helping sort out ions based on their charge and structure. The formula—C4H10O3S—might look plain, but it holds power to clean up complicated samples or help pharmacists figure out precisely what’s inside a batch of medication.
Lab nerds like myself remember how a bottle of 1-Butanesulfonic acid would sometimes get tucked into a locked cabinet, reserved for special applications. Sulfonic acids don’t just pop up in nature. Companies needed to synthesize them through chemical reactions involving butanes and sulfur trioxide or related compounds. These production routes require energy, attention to worker safety, and routines that keep environmental harm out of the picture. Modern guidelines, drawn up by agencies like OSHA and the EPA, stress proper handling and safe disposal. These measures protect lab staff and people living near chemical plants. Safety goggles and chemical gloves are more than fashion statements; they’re necessities.
Chemicals like 1-Butanesulfonic acid have become tools that shape entire industries. The trick lies in meeting demand without risking community health or the planet. I’ve watched as green chemistry ideas started taking root. Today, there’s growing interest in synthesizing sulfonic acids with methods that generate less waste and use renewable resources where possible. This shift matters beyond the lab. It lets us keep benefiting from compounds like C4H10O3S while reducing risks linked to older production processes. Regulatory frameworks hold companies accountable, but change also depends on scientists, workers, and even students pushing for safer chemicals and smarter practices.
Getting to know 1-Butanesulfonic acid means digging beneath that simple formula. Knowledge, safety, and sustainability work better together. Chemistry isn’t just for solving tricky equations or filling out reports—every formula impacts daily life, tracing real effects through what we eat, build, and treat. We have the tools to strike a balance between discovery and responsibility. By demanding stronger safety standards, backing sustainable manufacturing, and sharing what we learn, we make sure molecules like C4H10O3S help more than they harm.
I’ve spent enough years reading and reporting on chemical safety to spot common themes in industrial workplaces. Chemicals like 1-butanesulfonic acid rarely make the headlines, but anyone who has handled strong acids on the job knows that ignoring their risks can lead to trouble. 1-butanesulfonic acid, which acts as a surfactant, catalyst, and reagent in the lab, definitely comes with its share of hazards.
Most labels on these bottles warn about skin and eye irritation. Even just opening the container can sometimes make your nose itch. Inhaling the dust or vapors makes your throat scratchy, and skin contact can leave a rash, swelling, or even chemical burns. That’s not unique—strong acids usually act this way. But getting careless with this stuff can cost you more than discomfort. I once spoke with a lab tech who underestimated the irritation from simple splashes. It took days for her hands to heal, and she now wears gloves religiously.
It’s often easy to forget what happens after something flushes down the drain. 1-butanesulfonic acid dissolves easily in water and breaks down slowly. That makes wastewater treatment a challenge, because traces can linger and disrupt aquatic systems. Fish and microorganisms face big risks when acids like this enter the water. According to a 2020 review in the journal Environmental Toxicology and Chemistry, some sulfonic acids can persist and harm aquatic life for months. Those critters might look small, but their losses hit the food chain from bottom to top.
Factories and research labs pouring out liquid waste have to get this right. Disposal protocols aren’t optional—they’re essential. I’ve seen plant managers suspend work after accidental releases, then spend months fixing their reputation and footing the cleanup bill.
Toxicology studies don’t label 1-butanesulfonic acid as acutely toxic at the low exposure levels most workers face. The real problem comes from repeated exposure. Anyone spending eight hours a day in contact with it may face chronic irritation and even lasting respiratory problems. According to the European Chemicals Agency, evidence so far links long-term exposure with skin and eye damage but not outright mutagenicity or long-term organ effects. That may change, though, since research on some sulfonic acids is still young.
In my early days working next to chemical storage, the best defense always started with good habits: Gloves, goggles, and knowing where the eyewash station was. Training doesn't sound glamorous, but it gets results. New hires have fewer run-ins with harsh chemicals if the safety officer goes over the rules before anyone touches a bottle. Good labeling helps, too. Even experienced chemists can grab the wrong jug when things get busy.
Spills happen even with the best planning. Quick cleanup kits and clear procedures keep small accidents from growing. Environmental engineers now push for less hazardous alternatives and stronger treatment systems in wastewater plants. Simple steps—like monitoring pH at the drain and using neutralizers—cut risks for workers and downstream communities.
Chemistry moves fast. Some companies now substitute greener acids or use sealed systems to minimize worker and environmental contact. These efforts cost money up front, but they pay off when fewer employees get hurt and less toxic waste enters streams. I remember a plant in Ohio that switched to a less hazardous sulfonic acid blend; worker injuries related to skin exposure dropped by more than half over two years.
Nothing replaces careful handling, clear information, and respect for what a powerful acid can do. 1-butanesulfonic acid isn’t the most dangerous chemical you’ll find, but it deserves attention and respect from those who use it—at work and beyond.
Every time someone pulls out a bottle of 1-Butanesulfonic Acid, whether in a lab or a manufacturing site, they’re handling a chemical that has more bite than it might look. One careless move can bring on skin burns or even threaten the air with a sour, choking vapor. So storing this stuff isn’t about following an instruction manual—it’s about keeping workplaces safe and colleagues healthy.
There’s a reason even seasoned chemists double-check the shelf before stowing this acid. 1-Butanesulfonic Acid stands out as a strong acid, with the bite to corrode metal and gnaw through some plastics. If the container leaks, the acid can seep onto shelves or floors, react with other compounds nearby, and lead to chemical burns or toxic fumes. OSHA and EPA keep strict tabs on these risks for good reason. I’ve seen spills turn panic-worthy within seconds, even among folks who’ve handled acids for years.
Glass stands up well; high-density polyethylene does the job, too. Forget any temptation to use metal containers—even stainless steel won’t last long with this acid. Over time, inappropriate storage means eroded shelving or corroded seals, and then you’re facing leaks.
Always check that lids seal tight, not just after the first use, but every single time. Many labs label the acid with bold lettering, plus a date stamp. Handling the acid means thinking ahead—anticipating which container feels safe, and if the cap will hold during an earthquake or a simple knock from someone’s elbow.
Humidity and temperature make a big difference. 1-Butanesulfonic Acid prefers dark, cool spaces away from direct sunlight. Excessive heat can send pressure climbing inside the bottle, and even a well-sealed container might bulge or burst. I’ve seen summer heat in an unventilated storeroom warp bottles and loosen lids. At the same time, cold rooms offer a buffer, not just for the acid but for anyone fetching it—stable, under control, and less likely to react with stray moisture in the air.
Never store near bases, oxidizers, or organics. Mixing up storage shelves poses more risk than most realize. The Environmental Protection Agency documents plenty of chemical accidents that started with a storage mix-up. Separate acids from anything they might react with—a simple shelving plan can save headaches down the line.
Label shelves clearly. Simple signs—red borders for acids, green for bases—help stop mistakes, even for folks visiting the lab for the first time. Secondary containment trays catch spills. Some labs use plastic bins underneath each row of bottles, easy to clean and a second line of defense against drips or leaks.
Personal experience has shown that a twice-yearly review of the storage area works best. That means tossing old bottles, checking for crusted lids, and looking for signs of corrosion. Keep a spill kit nearby: neutralizer, gloves, and goggles all within arm’s reach. The sense of calm that comes with being prepared always beats the rush to clean up during an emergency.
Safe storage isn’t just about protecting a product—it’s about protecting people and the places they work. Good habits—checking labels, monitoring temperatures, keeping incompatible chemicals apart—have proven their value over decades. It all boils down to treating 1-Butanesulfonic Acid with the respect it earned by being both useful and hazardous, and understanding that a safe environment depends on daily attention, not just an annual review.
Everyone in a chemistry lab has faced the daily task of dealing with various acids, but some grab attention for reasons beyond common usage. 1-Butanesulfonic acid finds space on the shelf thanks to how it mixes physical reliability with chemical usefulness.
You pull the cap and find a colorless to pale yellow liquid. That clarity helps spot impurities fast—no surprises in the reaction mixture. In some conditions, it crystallizes, showing how temperature bumps can push its form one way or another. There’s an oily consistency, which isn’t unusual for sulfonic acids. Touching sulfonic acids teaches a lesson: gloves should always stay on. I still remember the sting from a careless thumb decades ago. The liquid isn’t runny like water—you notice more resistance when pouring.
That sharp, pungent odor lingers—a warning before the first drop hits the glass. The way this acid dissolves in water and lower alcohols makes sense. The “butane” part grants a touch of hydrophobicity, but the sulfonic acid group drags it into polar territory. No struggle dissolving in standard lab solvents, yet the acid doesn’t break into tiny pieces in straight hydrocarbons.
Melting sits around 87°C, so as room temperature shifts, you might face either a solid chunk or a puddle of yellow-tinted liquid by the end of summer. Boiling rides up past 300°C, so standard heating stirs up vapor but won’t boil it away easily. Those points aren’t just facts on a sheet; they shape storage choices in the lab and determine how you’ll handle spills or reactions that run hot.
Density tells a story about how it fits into solutions and how it layers during separations. Expect roughly 1.2 g/cm³, which feels heavier between the fingers compared to many organic liquids. The acid brings its strength to the mix: dropwise addition to water produces heat and aggressive fizz, always a sign of a strong acid. That pKa value, reported around -1.5, beats out many organic acids. Get careless, and the reaction vessel may remind you with some serious heat.
Dealings with sulfonic acids don’t finish with simply mixing solutions. Glass, PTFE, and most plastics shrug off contact, but store it in a metal flask and corrosion follows fast. Compatibility lists exist for a reason. I learned early to check lids and funnels before assuming all materials are created equal.
No commentary should skip over the risk. The acid eats through skin and can ruin clothing, not to mention the burns it makes on metal work surfaces. Handling protocols matter as much as the acid itself. Waste needs proper neutralization, and dilution steps save headaches later. Anyone with experience knows just a spill can cost an afternoon or worse—shut down an entire bench until clean-up finishes.
These physical properties change how scientists use and store the acid. Routine testing and mixing, sample preparation, and industrial purification all rely on knowing how the material behaves from the ground up. New users benefit from transparency—treat strong acids with respect, pick the right tools, and don’t ignore a change in appearance or smell. Physical traits don’t just fill tables in a datasheet; they guide safe, effective, and thoughtful work in the real world.
| Names | |
| Preferred IUPAC name | Butane-1-sulfonic acid |
| Other names |
Butane-1-sulfonic acid n-Butanesulfonic acid |
| Pronunciation | /ˈwʌn.bjuːˈteɪn.sʌlˈfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 5910-11-4 |
| Beilstein Reference | 1464774 |
| ChEBI | CHEBI:42561 |
| ChEMBL | CHEMBL1230443 |
| ChemSpider | 63100 |
| DrugBank | DB04115 |
| ECHA InfoCard | 100.008.503 |
| EC Number | 214-733-6 |
| Gmelin Reference | 6366 |
| KEGG | C01194 |
| MeSH | D016431 |
| PubChem CID | 10761 |
| RTECS number | EJ8750000 |
| UNII | WN1N1N9J8F |
| UN number | UN3261 |
| Properties | |
| Chemical formula | C4H10O3S |
| Molar mass | 150.21 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.26 g/cm3 |
| Solubility in water | soluble |
| log P | -1.1 |
| Vapor pressure | 0.06 mmHg (25°C) |
| Acidity (pKa) | -1.5 |
| Basicity (pKb) | -4.5 |
| Magnetic susceptibility (χ) | -48×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.425 |
| Viscosity | 3.08 mPa·s (25 °C) |
| Dipole moment | 2.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 130.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -860.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -859.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "P264, P280, P301+P312, P305+P351+P338, P337+P313, P330 |
| NFPA 704 (fire diamond) | 3-1-0 |
| Flash point | 163 °C |
| Autoignition temperature | 400 °C |
| Lethal dose or concentration | LD50 oral rat 1960 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 1960 mg/kg |
| NIOSH | WY0950000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.1% |
| Related compounds | |
| Related compounds |
Methanesulfonic acid Ethanesulfonic acid Propane-1-sulfonic acid Hexanesulfonic acid Tetradecanesulfonic acid |