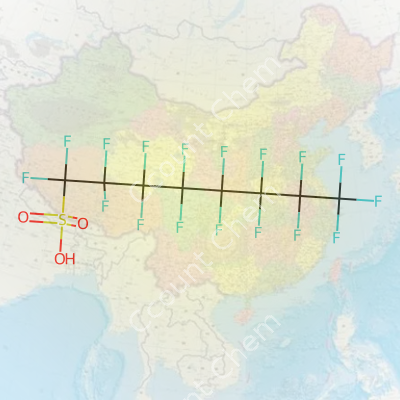
1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-Heptadecafluoro-Octane-1-Sulfonic Acid, often abbreviated as PFOS, entered labs and factories during a period when chemical companies chased water and stain-repellent finishes for everything from carpets to firefighting foams. By the late 1950s, large manufacturers rolled out PFOS derivatives, touting their stain-fighting power for consumer goods, paper, and industrial textiles. Regulatory oversight grew as scientists measured the chemical’s persistence in soil, water, and living bodies, fueling concern about unintended health impacts. Early excitement around its use gave way to a narrower focus as researchers documented how PFOS-resistant molecules built up in the environment. Experience shows that chemical innovation, left unchecked, often meets tough questions about long-term risk, both for people and planet.
PFOS stands as a perfluorinated compound, carrying a fully fluorinated eight-carbon chain topped off with a sulfonic acid group. Unlike short-chain alternatives that emerge in new consumer goods, PFOS lingers. It's been a favored additive in fire suppression agents, stain-resistant fabrics, and plating baths for chrome industries. Surfactants built on PFOS bring stability and non-stick ability without giving in to heat or solvents, which made it irreplaceable in a ton of specialties before tighter regulations clamped down. My years reading scientific reports made it clear: once PFOS goes in, it stays—and that stubbornness created a legacy issue in water and wildlife.
Heptadecafluorooctanesulfonic acid looks like a white powder or off-white granules at room temperature, with a faint, almost medicinal odor that points to its inorganic strength. It dissolves in organic solvents, but water does not break it down readily, letting it move through aquatic systems unchecked. With a high boiling point that allows it to survive in extreme temperatures, PFOS resists acids, bases, and most oxidizers. Its surface tension-lowering effect proved handy in forming robust coatings. The carbon-fluorine bond, one of the tightest in chemistry, leaves PFOS standing after other compounds break apart, a feature which sadly leads to its label as “forever chemical.”
Bottled or bagged industrial PFOS comes with a CAS number, UN proper shipping name, and GHS hazard pictograms on every label. Most chemical safety sheets reveal its molecular weight, boiling point, melting point, purity, and recommendations for safe handling. In my practical work, regulatory paperwork matters just as much as barrel specs. Many producers set purity at 95% or higher, as impurities trigger increased risk during reactions. Maximum allowable limits set for transport by air or sea force manufacturers to monitor quality in every step from synthesis to delivery. Sometimes, label warnings feel remote, but with PFOS, strict labeling offered the first line of defense.
The main route for synthesizing PFOS draws on electrochemical fluorination of octanesulfonyl fluoride, passing electric current through a mix of hydrogen fluoride and precursor hydrocarbons. This process swaps hydrogen atoms for fluorine straight down the carbon chain, creating even distribution and chemical stability. Some labs tried telomerization, but found that only full perfluorination delivered the acid’s blend of durability and surfactant properties. Each batch can bring byproducts and impurities—something that affects performance and hammers home the importance of post-reaction purification. Personal observation: few methods match the old-school electrochemical fluorination for yield and scope, but modern pushes toward green chemistry call for safer alternatives.
PFOS rarely breaks under routine chemical reactions. A mix of stubbornness and resilience makes it tough to degrade, even using aggressive chemicals or heat. That spells issues for remediation. Chemists know you can modify the sulfonic acid group to generate salts or esters; potassium and ammonium salts find use in specialty chemical industries. Attempts to crack or defluorinate PFOS in labs often demand high-temperature reactors or powerful reducing agents—approaches not feasible outside controlled facilities. Recent research explores photocatalytic and enzymatic breakdown, but those processes can move at a crawl. The chemical grip of the carbon-fluorine bond separates PFOS from more easily treated industrial acids, complicating both environmental cleanup and waste treatment.
Many know this compound under aliases like perfluorooctane sulfonic acid, PFOS acid, or FC-95. Commercially, it appeared under listings such as Fluorad, Surfonic PFOS, and Teflon-based stain protectors. Each name marks a history tied to brand identity, but under the hood, the chemistry stays unchanged. Reading regulatory filings, one notices how international labeling requirements force companies to adopt every synonym, making it clear that chemical memory runs long in global supply chains.
Years of gathering regulatory evidence shaped best practices for handling and disposal. Workers must wear gloves, splash goggles, and chemical aprons. Tightly sealed containers prevent vapor drift and spills, while exhaust systems purge airborne particles during transfer and mixing. Emergency showers and spill kits become more than mere box-ticking—they stay in reach for a reason. Companies moving PFOS across borders supply detailed MSDS with hazard codes, exposure limits, and toxicity profiles. My own work in labs brought home just how fast skin exposure can happen, and why every handler needs proper fit-tested respirators. More operations now audit PFOS procedures regularly, but old sites still lag behind, posting ongoing risks for accidents or leaks.
Firefighting foams based on PFOS set the standard for airport fire safety decades ago; few alternatives offered the mix of film-forming and re-ignition resistance. Metal plating shops counted on PFOS wetters to stop hexavalent chromium aerosols blanketing shop floors. In textiles, stain-proof uniforms benefited from a coating of this fluorinated acid, helping workers fight off chemical splashes and food stains. Electronics manufacturing tapped PFOS salts for etching agents in semiconductor processing, where cleaner lines meant higher yields. Print houses leaned on it as a surfactant for bright, crisp inks. Over time, each sector learned that short-term breakthroughs in performance brought long-term questions about health and legacy contamination.
The push to phase out PFOS reshaped research priorities across chemical engineering, environmental science, and medical studies. Scientists track PFOS in blood, liver, and drinking water, often comparing overall exposure to regulatory thresholds set by national and international bodies. Research papers probe its effects on immune response, liver enzymes, and hormone regulation. Meanwhile, industrial labs pivot toward alternative surfactants, designing molecules that retain performance but break down more readily in the environment. Success feels mixed; new molecules sometimes bring lower efficacy, new toxicity worries, or higher costs. Years spent farming journals for new data made one thing clear: balancing utility and safety means ongoing trial—there’s no shortcut to safe chemistry.
Animal studies record bioaccumulation in liver, kidney, and serum. Researchers link chronic PFOS exposure to cholesterol spikes, thyroid disruption, and immune suppression, both in the lab and, increasingly, in population-based studies. Regulatory bodies around the world, from the US EPA to the European Chemicals Agency, now rank PFOS as a persistent organic pollutant with confirmed toxicity in mammals and aquatic life. Experience shows that untangling human health impacts takes time and care—epidemiological findings shift slowly, especially for compounds that build up silently over decades. Monitoring of breast milk, tap water, and occupational samples tells a complex story shaped by both concentration and duration. While toxicologists fill in gaps, public health experts recommend minimizing new releases and funding long-term tracking studies.
Hopes for safer chemistry ride on both cleanup technologies and green synthesis pathways. New sorbent materials show promise for extracting PFOS from wastewater, but handling and disposal of contaminated filters still loom large. Bioremediation trials tap into specialized bacteria and enzymes that nibble away at the carbon-fluorine bond, though no field-scale success stories yet match conventional incineration. Policy shifts press industry to drop PFOS from supply chains, even as legacy contamination drives cleanup budgets ever higher. Companies and research groups chase alternatives with shorter chains and reduced bioaccumulation, but the test comes in their actual persistence and toxicity—a lesson learned hard in PFOS’s wake. Public awareness climbed as documentary coverage and investigative reports hit the mainstream, triggering calls for chemical transparency and tighter stewardship at every stage from synthesis through end-of-life. Experience puts the lesson plainly: chemistry shapes health and habitat for years to come, so care up front saves trouble for generations after.
This tongue-twister of a molecule, often called PFOS or perfluorooctane sulfonic acid, doesn’t pop up in daily conversations. The name might look strange, but the uses have shaped modern manufacturing and still cast a shadow over today’s environmental scene. Growing up surrounded by news stories of Teflon pans, stain-resistant carpets, and that weird chemical smell in new hiking gear, I realized—much later—just how many products trace their “don’t get wet, don’t stick” qualities back to chemicals like this one.
PFOS shines as a surfactant. It lets water, dirt, and oil slide right off surfaces where most things would stick. In my old job at an industrial laundry, we sprayed uniforms and tablecloths with stain-resistant treatments. PFOS and its cousins often powered that effect. Instead of soaking up coffee stains, those tablecloths just shrugged messes off. Firefighters’ foam used it for fast-spreading action across burning liquid fuel. Back in the day, big electronics manufacturers counted on PFOS to keep circuit boards clean during production, since the molecule stood up to harsh conditions unlike anything else.
After years of use, problems came to light. PFOS doesn’t break down easily. It builds up in blood, wildlife, and water. Scientists started finding it far from factories, showing up in fish and even polar bears. The U.S. EPA noted high levels could hurt the liver and immune system, raising red flags for some kinds of cancer. That hit home when my local water department flagged potential PFOS contamination from an army base. News spread, trust drained out of the taps, and bottled water became the short-term fix. The fact that PFOS holds on where it lands—a river, a field, or a body—is key to why so many communities worry about this chemical group.
Some companies still stick with PFOS, mainly in specialized photolithography and certain heat- and corrosion-resistant coatings, where few other molecules perform as well. The chemical doesn’t just slide away from strict bans because of industry loyalty; the replacements either cost more, don’t work as well, or bring their own unknowns. Think jet fuel spill fires or critical electronics—a substitute must prove itself just as tough. The loss of PFOS in many firefighting foams left emergency crews, airport managers, and oil field safety teams scrambling to adjust training and inventory. For things like microchip manufacture, switching chemistry takes time, experimentation, and money.
Cleaning up after widespread PFOS use will run for years. My region is looking at enormous bills for water treatment upgrades, and families near older factories want accountability. Research now focuses on how to destroy these molecules—high-pressure, high-temperature reactors that chew up the bonds, or bacteria genetically tweaked to do the hard work. Manufacturers shy away from designing new “forever chemicals,” turning to more biodegradable surfactants even if they fall short on toughness. Legislation around the world tightens each year. As stricter testing comes into play, companies find themselves back at the drawing board, and communities push for more transparency about chemicals in local air and water.
1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-Heptadecafluoro-octane-1-sulfonic acid belongs to the family of PFAS. Scientists and regulators usually call these “forever chemicals” because they don’t break down in the environment. I’ve seen the headlines over the years, watched the documentaries, and listened to neighbors worry about tap water. PFAS stick around long after most other pollutants fade away. Some researchers say even a few parts per trillion can stay in the body for years.
People living near chemical plants or airports know all too well what it’s like to learn their water tests positive for PFAS or similar compounds. A large body of studies links this group of chemicals to health issues. The ones that stand out come from work by the US Environmental Protection Agency and National Institute of Environmental Health Sciences, which tie exposure to increased cholesterol, shifts in liver function, problems with hormones, and a higher risk of some cancers. A 2012 study in the journal “Environmental Health Perspectives” linked PFAS exposure in children to low immune response following vaccination.
I remember a small town in Michigan facing a wave of mysterious thyroid issues. Residents there measured PFAS levels that made national news. Doctors couldn’t point the finger at anything except the water everyone relied on. It made me wonder, what really qualifies as “safe” exposure to these chemicals? When officials revise the safety thresholds downward almost every year, it creates doubt about old assurances.
This specific acid isn’t just a hidden lab compound. Industries used compounds like it for decades in products that seemed harmless: stain-resistant carpets, firefighting foams, water-repellent coatings on clothes, even some fast-food wrappers. Most people never gave a second thought to how those stains would roll right off a shirt—until high-profile lawsuits pulled PFAS into the spotlight.
The US Centers for Disease Control and Prevention found PFAS in the blood of almost everyone they tested. These chemicals travel, not just sticking to soil, but contaminating rivers and groundwater where the reach goes far beyond the original factory fence. Fish pick it up, so does wildlife, and eventually, families face it at the dinner table.
No one can just wish PFAS away. I learned from years watching local governments struggle with contaminated wells that the fixes rarely come cheap or quickly. Activated carbon filters, high-tech reverse osmosis, and digging new wells all carry big costs. Communities with less money feel the impact harder.
Policymakers set lower limits for PFAS in drinking water, which forces utilities to act. Stronger chemical regulations will slow down further pollution, but they won’t magically clean up what’s already settled in the water and soil. People can test their water and use filters at home, but no single household can fight this alone.
Scientists keep studying how PFAS damage the body and tracking how every new chemical relative works its way through water, air, and food. Keeping informed and pushing for better industry oversight shapes what happens next. If this generation learns from these chemical lessons, the next one might not be stuck cleaning up another unseen mess.
Working around chemicals changes how you move and think. A label with a warning means paying real attention—one slip and you’re racing to the eye wash or worse, the ER. I remember the first time I handled concentrated sulfuric acid in a college lab, hands trembling inside those thick gloves. The memory of that sting pushes me to remind others: safety gear stands between you and permanent injury.
Most people spot the white coats and goggles in movies and think it’s all for show. In reality, even a couple splashes can melt holes through clothes and burn skin. Proper gloves—nitrile, not the cheap vinyl kind—and goggles with a tight seal keep splashes off your eyes and hands. Long sleeves and closed shoes don’t seem exciting until you spill something and see the socks fizzle. Good gear costs a little more, but losing vision or burning your hand is permanent.
Sometimes a chemical smells sweet or sharp, letting you know it's in the air. Many gases don’t warn you at all—think of colorless vapors from cleaning solvents turning a closed room into a trap. Fume hoods can look clunky, but they clear out harmful air before it creeps into your lungs. Fans and open windows help, but only a proper hood guarantees the air won’t carry dangerous vapors.
Each bottle comes with symbols and warning words, but too many folks brush past the data sheets. A Safety Data Sheet (SDS) gives instructions for spills, emergency care, and storage that aren’t obvious just by looking. One wrong guess—say, adding water to a reactive powder—can trigger explosions or poisonous vapors. Before touching unfamiliar chemicals, scanning that SDS makes the difference between a close call and a disaster.
There’s no way to prevent every spill, no matter how careful you work. Absorbent pads, sand, and neutralizing agents need to sit close, not hidden at the bottom of a supply closet. Proper containers keep waste from leaking. I’ve seen too many folks pour leftovers down the drain, which pollutes waterways and risks chemical reactions inside pipes. Taking time to read up on disposal means safer water and easier lab inspections.
Companies hold safety meetings for a reason—music to nobody’s ears, but those drills kick in when accidents happen. Knowing where the eyewash station sits doesn’t help unless you can reach it blindfolded. Clear, frequent training means nobody stands guessing during a real emergency. Nobody wants fire extinguishers collecting dust while staff run for the exits.
Eat and drink far from working areas. Wash hands, even with gloves on all day. Don’t rush to finish early—speed leads to spills, confusion, and missed warnings. In the end, respecting the risk keeps everyone safe, including coworkers, cleaning staff, and the community outside the lab’s doors.
Some chemicals just aren’t made for careless storage. One of them is 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-Heptadecafluoro-Octane-1-Sulfonic Acid, a strong fluorinated compound showing up in labs and some manufacturing spots. This mouthful of a substance doesn’t only require thick gloves. People need a reliable plan for how and where it waits before use. My years around labs have drilled home that error usually doesn’t announce itself with flashing lights. It creeps in through shortcuts. Careless storage means a risk for the person working, anyone walking past, and, if it leaks, the environment outside.
Most folks glance at a chemical label, see terms like “fluorinated” or “sulfonic,” and toss the bottle onto the closest shelf. This one demands more thought. Its strong acidity and stability—yes, those “forever chemical” worries—mean glass, high-grade stainless steel, or fluoropolymer containers work. Everyday plastics don’t stand much of a chance. Polystyrene and polyethylene degrade, and no one wants cleanup duty after a spill so nasty. People sometimes cut corners, putting savings over safety. History shows that doesn’t end well. For chemicals like this, I never try to reuse questionable containers or let them share space with reactive stuff like strong bases or metal powders.
Shelving always says something about priorities. Storing this chemical at room temperature in a dry, cool, well-ventilated spot gives the best results. Locked cabinets marked clearly keep accidents in check. I always make sure acids never bunk with bases, oxidizers, or fuel sources. The stuff stays sealed tight, away from edges where it could be knocked over or get bumped. In labs short on space, trying to group chemicals for “just a night” invites trouble. Security isn’t just a lock. It’s controlling who knows what’s in the cabinet, how much sits there, and the last date someone checked its label and integrity.
Store it wrong, and health and environmental risk climb fast. This acid can linger in air, water, and soil much longer than most. The link between fluorinated compounds and chronic health problems isn’t abstract. Studies show bioaccumulation, liver and immune system impact, and the risk of water contamination at very low concentrations. News stories from communities near chemical plants paint a clear picture. An accident, lazy storage, or unnoticed leak lets those problems move outside the lab.
Standard operating procedures bring order. Frequent checks for signs of wear on containers, clear labeling (including hazard ratings), and inventory logs sorted by date help spot risks early. In my experience, direct training—walk-throughs over long policy sheets—sticks with people. Staff absorb what hands-on means: Don’t move a container if you haven’t checked for leaks or pressure, don’t leave doors open, and don’t rely on memory for handling steps. Emergency kits with absorbents and protective equipment close at hand beat hunting through dusty closets after something spills. Keep spill emergency guidance visible.
Proper storage isn’t just a checklist. It’s respect for coworkers and the towns and rivers nearby. Actions in the lab shape safety everywhere else. With chemicals like 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-Heptadecafluoro-Octane-1-Sulfonic Acid, true diligence doesn’t just prevent fines or regulations. It earns trust and protects everyone’s future. Experts with experience in similar environments, up-to-date scientific data, and real-world results have shown again and again: proper storage beats cleanups every time.
Every country takes a different approach to regulating chemicals because public safety, economic pressure, and political priorities shape decisions in unique ways. I’ve spent time in both the US and Europe, so I’ve watched how this affects products on store shelves, medical treatments, or even cosmetics you trust at home. Walking into a pharmacy in France, you’ll often discover ingredients banned just across the Channel in the UK, or vice versa. The rules are rarely universal.
You rarely find strict rules announced in a vacuum. Usually, a high-profile health scare or scientific breakthrough gets people asking questions. Once an investigation finds risks—harmful to breath in, unsafe for workers, long-term cancer connections—regulators step in. The European Union uses the REACH legislation, which stands for Registration, Evaluation, Authorisation, and Restriction of Chemicals. REACH keeps a record of thousands of chemicals. If scientific evidence points to a threat, they list the compound as restricted or even outright ban it.
In North America, the US Environmental Protection Agency tracks dangerous substances under the Toxic Substances Control Act. Canada keeps their own Domestic Substances List. These agencies can require special handling, license limits, or list certain compounds as illegal for sale. The world saw this happen with asbestos, leaded gasoline, and certain industrial solvents. Sometimes, what’s seen as too risky in one country might still show up in a less regulated market. That double standard tends to drive black market trade or encourage loopholes.
Manufacturers don’t want complicated systems that stop their products from moving easily between countries. When regulators add a compound to the “restricted” list, there’s backlash. It comes from lobbying, lost jobs, or even angry consumers. The sunscreen debate has gone global—oxybenzone and octinoxate got banned in Hawaii to protect coral reefs. Now, the personal care industry scrambles to find safe alternatives that work and pass new safety tests. People want both environmental protection and dependable products.
A big problem comes up when restrictions confuse consumers. I’ve run into parents who thought buying a children’s toy in one country meant it was safe everywhere. But regulations change with each border. That’s why certain plasticizers, like phthalates, disappear from products in Europe but linger in the US market—or vice versa. Folks deserve to have plain-language updates so they don’t feel betrayed by what’s available at home.
Fixing the gaps between regulations leads back to better cooperation. If scientific data on a certain chemical’s risk stacks up, safety should apply no matter where it’s sold. International standards groups—like the World Health Organization or the OECD—try to create common frameworks. More countries joining in can help shrink the gaps and cut down on unsafe imports sneaking through.
Public transparency stays at the core—when governments explain why rules change, and when companies tell shoppers what’s in their products, trust grows. If you’re ever unsure about a compound’s legal status, look up the government lists in your country and check with respected health agencies. Local and global efforts both matter, but staying informed protects both families and business. Experience tells me consistent, open communication saves lives and keeps people from learning too late that a risk was preventable.
| Names | |
| Preferred IUPAC name | 2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-Pentadecafluorooctane-1-sulfonic acid |
| Other names |
Perfluorooctanesulfonic acid PFOS Heptadecafluorooctanesulphonic acid Perfluorooctane sulphonate Perfluorooctylsulfonic acid |
| Pronunciation | /ˌhɛp.təˌdiː.kəˌfluːəˈrɒk.teɪn.wʌn ˈsʌl.fə.nɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 1763-23-1 |
| 3D model (JSmol) | `JSmol.loadInline("data/mol/PFOS.sdf")` |
| Beilstein Reference | 3568941 |
| ChEBI | CHEBI:38941 |
| ChEMBL | CHEMBL4295697 |
| ChemSpider | 10805218 |
| DrugBank | DB11357 |
| ECHA InfoCard | 03bb6b62-27b5-408d-bea3-08e97a2cdf3a |
| EC Number | 3.1.6.21 |
| Gmelin Reference | Gmelin Reference: 84054 |
| KEGG | C19587 |
| MeSH | D020110 |
| PubChem CID | 67622 |
| RTECS number | GU9625000 |
| UNII | 41P2W8GX6I |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID2021362 |
| Properties | |
| Chemical formula | C8HF17O3S |
| Molar mass | 538.14 g/mol |
| Appearance | String: C8F17SO3H |
| Odor | Odorless |
| Density | 1.86 g/cm3 |
| Solubility in water | Solubility in water: 570 mg/L (25 °C) |
| log P | 5.3 |
| Vapor pressure | 1.74E-4 mm Hg at 25°C |
| Acidity (pKa) | -3.3 |
| Basicity (pKb) | -4.22 |
| Magnetic susceptibility (χ) | -0.62e-6 cubic centimeters per mole |
| Refractive index (nD) | 1.306 |
| Viscosity | 500 cP |
| Dipole moment | 3.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 527.2 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1715.7 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1793 kJ mol-1 |
| Pharmacology | |
| ATC code | J06BA01 |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS05, GHS07, GHS08 |
| Pictograms | GHS05,GHS07,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H302, H314, H361fd, H373, H411 |
| Precautionary statements | Precautionary statements: "P261, P273, P280, P304+P340, P305+P351+P338, P312, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0-W |
| Flash point | > 115 °C |
| Lethal dose or concentration | LD50 (oral, rat): >2000 mg/kg |
| LD50 (median dose) | > 2000 mg/kg (rat, oral) |
| NIOSH | GN0107000 |
| PEL (Permissible) | 0.1 mg/m3 |
| REL (Recommended) | 0.0005 mg/L |
| Related compounds | |
| Related compounds |
Perfluorooctanesulfonyl fluoride Perfluorooctanoic acid Sulfanilic acid 2,2,2-Trifluoroethanesulfonic acid |