Chemists have walked a long road to reach the current understanding and applications of perfluorinated compounds. The journey began with the widespread interest in organofluorine chemistry during the mid-20th century, spurred by discoveries on the unique stability, chemical resistance, and hydrophobic properties of fluorinated molecules. Early research focused on perfluoroalkyl sulfonyl fluorides, a class known for their robust bonds and unique reactivity. Notably, scientists channeled efforts into developing nonafluorobutane-1-sulphonyl fluoride as a specialty derivative, driven by the need for highly reactive, yet stable, sulfonyl transfer reagents. Years ago, the lab work needed custom syntheses, but modern availability greatly changed the scene—manufacture scaled up, and researchers moved beyond mere curiosity into practical fields like pharmaceuticals, agrochemicals, and surface chemistry. Each leap in synthetic method brought clearer insight. The legacy of foundational research continues: tough regulations and keen interest in environmental impact have sharpened focus lately, challenging companies to innovate with purpose and care.
1,1,2,2,3,3,4,4,4-Nonafluorobutane-1-sulphonyl fluoride stands out as a fluorinated sulfonyl compound featuring nine fluorine atoms arranged along a four-carbon chain with a terminal sulfonyl fluoride group. In my work with synthetic labs, this compound has played a central role in sulfonylation and as a precursor in fluorinated surfactant development. It brings significant value to chemists due to high reactivity, ease of handling in controlled environments, and powerful electron-withdrawing effects from its heavily fluorinated backbone. Chemically, it bridges the gap between performance and functionality, with strengths that push beyond other sulfonyl fluorides.
The molecule's physical presentation usually consists of a colorless, low-viscosity liquid. Its melting point remains markedly low, making storage and transfer straightforward under cool, dry conditions. Boiling point sits above many analogous sulfonyl fluorides, a direct result of fluorination, which brings both chemical inertia and volatility. Immense electronegativity from the fluorine atoms shields the carbon chain from nucleophilic attack; water and acids scarcely dent its structure. I’ve noticed colleagues always comment on its excellent thermal stability, which permits broader reaction windows when pursuing demanding synthetic work. The molecule shows little affinity for hydrogen bonding, so it floats easily through largely organic phases. Under typical conditions, it releases pungent fumes if decomposed, a clear sign of both potency and required care.
Manufacturers label nonafluorobutane-1-sulphonyl fluoride with potent hazard signs: corrosive, toxic by inhalation, and environmentally hazardous. In practical settings, chemical supply sheets detail a minimum purity of 98%, listing residual perfluorinated byproducts and water content down to parts per million. Storage guidelines emphasize shielding from heat, sparks, and moisture—a dry, inert atmosphere works best. Bottles carry tamper-proof seals and secondary packaging to prevent leaks, which speaks to the trust needed between supplier and end user. Detailed lot analysis and chain of custody secure product accountability, aiding in lab audits and regulatory compliance.
Synthesis starts with perfluorobutanesulfonyl chloride as a key intermediate. Fluorination, often through electrochemical fluorination (ECF) or direct fluorine gas treatment, converts butanesulfonyl chloride into the fully fluorinated backbone. This step charges the molecule with remarkable chemical stability. Next, the sulfonyl chloride group undergoes substitution with fluoride sources—commonly, anhydrous hydrogen fluoride or potassium fluoride—yielding the final product. Each stage calls for skilled technicians, as control over temperature, pressure, and purity matters greatly. From a practical standpoint, waste streams require strict scrubbing and neutralization routines, given the hazardous byproducts.
Chemists deploy this molecule primarily in sulfonyl fluoride transfer reactions, using it to install sulfonyl fluoride moieties onto more complex frameworks. Exposure to nucleophiles (alcohols, amines, or organometallics) produces tailored sulfonates or sulfonamides, expanding the chemistry possible with perfluorinated motifs. Reductive and substitution chemistry often figure in protocols, as well as coupling strategies with arene systems. The unique electron-deficient chain makes it a versatile partner in cross-coupling, stabilizing reactive intermediates and minimizing side reactions. Tweaks to the backbone enable researchers to explore structure-activity relationships, critical in drug discovery where such groups lend metabolic resistance.
Literature and catalogs often refer to this chemical as “Nonafluorobutane sulfonyl fluoride,” “Perfluorobutanesulfonyl fluoride,” or simply “NfBSF.” The CAS registry number removes ambiguity. Some industrial suppliers print alternative names on labels to match global nomenclature systems, reflecting regulatory expectations across markets. This mishmash of synonyms occasionally brings confusion to non-specialists, so precise identification tools and cross-referencing with Chemical Abstracts Service databases go a long way in preventing mix-ups.
Safety protocols matter in all areas of perfluorochemicals, and with nonafluorobutane-1-sulphonyl fluoride, the stakes feel even higher. Direct contact causes burns or blistering, and inhalation irritates respiratory tracts. In our lab, training covers not only PPE (nitrile gloves, goggles, lab coats), but also specialized chemical fume hoods, splash shields, and spill kits tailored to aggressive fluorinated agents. Technicians get clear-cut evacuation instructions, and management reviews response plans every quarter. Fire suppression measures avoid water—those who’ve seen vigorous exothermic reactions never forget that lesson. Spills trigger high alert: containment, careful neutralization, and professional disposal align with modern waste regulations. Long-term, storage focuses on minimizing exposure risk to both people and the environment, a balancing act that shapes workflow design.
Perfluorobutanesulfonyl fluoride influences several important sectors. In medicinal chemistry, it builds pharmacophores with fluorinated sulfonyl moieties, extending drug lifetime or modulating metabolic breakdown. Agrochemical research also benefits: adding sulfonyl fluoride groups to pesticides or herbicides often tunes bioavailability and enhances environmental stability, or in recent times, helps manage resistance. The electronics sector exploits the compound’s non-stick and high-dielectric properties when tweaking surfaces or insulating microchips. Manufacturers often apply it in custom syntheses—to fluorinate surfactants or lubricants, pushing performance under harsh industrial conditions. My experience with research institutions points to growing use in probe design, especially tools for tracking protein activity—an expanding corner of bioorthogonal chemistry.
Scientists, industrial chemists, and regulatory bodies all see the R&D field around this compound evolving rapidly. Innovation centers on cleaner, more efficient synthesis pathways: minimizing hazardous reagents, improving atom economy, and cutting energy costs. Greener chemistry approaches—less solvent, condensed reaction steps—shape the current landscape. Academics analyze the molecule’s electronic features, seeking modifications that balance reactivity with biocompatibility. In my own projects, collaborative work between academic and industrial teams continues to uncover new catalyst systems and expand the scope of functional groups tolerated during late-stage modifications. The patent literature bears this out: filings climb steadily, pointing to interest in polymer chemistry, next-gen coatings, and diagnostics. Regulatory shifts now push researchers to track fate and transport in water and soil, prompting environmental science input and deeper toxicological studies.
Any commentary on perfluorinated agents must face the question of toxicity head-on. Nonafluorobutane-1-sulphonyl fluoride, while stable in matrices, raises red flags for bioaccumulation and long-term persistence. Animal testing shows potential for liver and reproductive toxicity at medium to high doses, and even trace environmental releases accumulate over time, both in water tables and organisms. Early-run studies flagged subtle immune effects, spurring regulators to demand meticulous data. From the vantage of modern science, this has led to a shift in risk assessments: routine worker biomonitoring, stricter handling controls, and periodic review of permissible exposure levels. Analytical chemists have improved detection, catching minute concentrations in air, soil, or effluent streams. Toxicity research still lags behind production pace; this gap keeps pressure on both industry practitioners and public agencies to invest in transparent, reproducible science.
Looking ahead, nonafluorobutane-1-sulphonyl fluoride will not disappear from advanced chemical toolkits. Demand grows for new sulfonyl fluorides, but market shifts and regulatory landscapes challenge continued use. Companies invest more heavily in green chemistry, eyeing alternatives with lower persistence, shorter biological half-lives, and equal impact for reaction engineering. The molecule likely sees further tuning, so future variations keep up with both scientific and environmental demands. Ongoing research should clarify safe handling and disposal paths, and improved risk mitigation plans ought to filter out unsafe legacy practices in factories and laboratories. Innovation will have to walk that line—maintain the power of fluorinated reagents, but carve out a trail that fits a safer, more accountable kind of chemistry for the next generation.
1,1,2,2,3,3,4,4,4-Nonafluorobutane-1-sulphonyl fluoride can sound as intimidating as a chemical formula gets. At its core, it belongs to a class called perfluorinated sulfonyl fluorides. These aren’t household names, but for research chemists and industrial folks, compounds like this one make a difference.
Over the last few decades, fluorinated chemicals have stolen the show in materials science and drug development. I remember a friend in a pharmaceutical lab explaining the chase for molecules that do two things: deliver a reaction and survive harsh conditions. Nonafluorobutane sulphonyl fluoride fits the bill. It emerges as a stellar starting point for making reagents called “nonaflates.” In the lab, these nonaflates help swap out parts of carbon rings—a little like changing out tires on a car so it runs differently.
Its claim to fame comes from how easily it marks alcohols to turn into these nonaflate groups. These groups jazz up the molecules for reactions called cross-coupling. The Suzuki and Stille reactions depend on nonaflates to build lots of useful compounds—everything from agrochemicals to active pharmacy ingredients. The upshot? One odd-sounding chemical unlocks new molecules that tackle disease or boost crop yields.
Beyond the research bench, nonafluorobutane sulphonyl fluoride steps into roles tied to manufacturing specialty materials. Some companies use it to prepare tailored polymers or fluorinated coatings. With each generation of battery or microchip, clean, robust chemistries keep manufacturing lines humming. Fluorinated sulphonyl fluorides contribute to polymers that resist breaking down in harsh industrial environments. Those properties help electronics last longer and perform better under tough conditions.
Fluorinated chemicals draw both praise and concern. On the one hand, they build molecules with impressive stability. A classic case comes from non-stick cookware, fuel cells, and even firefighting foams. On the other, worries have grown over so-called “forever chemicals”—perfluorinated substances hanging around in the environment. Nonafluorobutane sulphonyl fluoride, with its nine fluorines, stacks up as a potential environmental challenge if used carelessly.
Personally, working in a university setting, we used to treat these reagents with extra care. Fume hoods, proper gloves, clear waste segregation. In bigger plants, strict disposal and containment rules apply. Regulatory agencies now expect detailed record-keeping and push for greener alternatives when they exist.
Research keeps moving fast in this field. Some chemists chase after less persistent alternatives: shorter-chain fluorinated versions, or even fluorine-free routes that mimic the same effects. Industry has also boosted recycling—the same nonafluorobutane sulphonyl fluoride sometimes reclaimed after a reaction, distilled, and reused. That takes some heat off disposal worries.
Stronger oversight from public agencies and greater transparency from companies help, but the best changes come from the ground up: safer lab habits, smarter process design, and a willingness to rethink old formulas. As we keep unlocking new medicines or materials, the choices we make with these building blocks echo far beyond the bench.
Dealing with chemicals demands simple routines built on respect and routine, not anxiety or panic. Take, for example, household bleach—something most families keep under the sink. Its label warns of eye and skin irritation, so no one should treat it carelessly, just as folks wouldn't juggle knives in the kitchen. The principle remains the same for industrial-strength acids or volatile solvents. In my experience, the first instinct—“How do I keep this away from my eyes, skin, and lungs?”—remains reliable, no matter the setting.
Every time I’ve handled a chemical, gloves, goggles, and closed-toe shoes never seemed optional. During college chemistry labs, a classmate splashed sodium hydroxide near his wrist. He wore long sleeves and gloves, so he only suffered a small scare instead of a nasty burn. These basics save injuries. Nitrile gloves usually stand up to oils and a lot of solvents. Goggles block surprise splashes. Even a simple lab coat limits how much goes straight onto skin or regular clothes. This gear means fewer emergencies and less time rinsing off chemicals under a safety shower.
Breathing fumes never ends well. Ammonia, chlorine, acetone—the list goes on—send sharp reminders up your nose and into your lungs if air circulation falters. I once volunteered in a cramped storeroom and found out fast that even mild acids need more than just cracked windows if people mix or pour them inside. Fume hoods, or at least strong exhaust fans, push trouble away from your face and everyone else nearby. In homes, even just stepping outdoors for heavy-duty cleaners makes a difference. According to the CDC, poor ventilation counts as a top source of chemical-related illness in homes and workplaces.
Pouring a chemical into a coffee mug or juice bottle tempts fate. Wrong containers lead to mix-ups and real poisonings—especially with kids or distracted adults nearby. Labels matter. I’ve watched people stick painter’s tape on everything, scribbling the contents and the date. It works. Flammable solvents belong in metal cabinets, acids apart from bases, oxidizers in their own marked space. The National Fire Protection Association paints a clear picture: store like items together, and never underestimate the value of a locked cabinet.
Skipping the fine print brings bigger headaches later. Chemical manufacturers put time into those instruction sheets for a reason. They outline what to do if someone accidentally swallows or spills something—steps I have actually seen save someone from making a mess worse. Reviewing what a product does, checking its warning symbols, and planning ahead for accidents brings peace of mind and keeps everyone safer.
Whether at work, home, or school, taking even minor precautions around chemicals builds a safer routine. The American Chemical Society and the Occupational Safety and Health Administration provide guides that translate well to daily life. Protecting the skin and eyes, keeping a window open, labeling containers, and reading product labels do not take extra training. They give anyone a real shot at reducing accidents and staying healthy, lesson after lesson, day after day.
Every time a new chemical product turns up, someone in the lab or on the purchasing side wants to know, “What’s the molecular formula?” No surprise there—it answers real questions. The formula is a snapshot, showing every type of atom in a compound and how many of each show up in a pile of those molecules. Take ethanol for example. C2H6O shows two carbon atoms, six hydrogen atoms, and one oxygen atom in every single molecule. The formula tells chemists what’s inside, plain and simple.
Details in that formula matter even when things look similar at a glance. Isopropanol has the same molecular formula as propanol, C3H8O, but a different arrangement of atoms. Even so, front-line questions—safety, environmental behavior, or cross-reactions—always start by checking that formula. It’s the ID badge that helps you steer clear of mix-ups and mistakes.
Once you know the formula, the next ask usually points to the molecular weight. Some see it as a dry number. That number, though, shapes nearly every calculation that follows in the lab. Let’s say someone is preparing a buffer or buying chemical stock. They need precise quantities or concentrations for reactions to go as planned. Even a simple conversion between grams and moles depends on molecular weight. A single wrong digit could mess up purity, waste expensive chemicals, or trip up an experiment’s results.
Out in manufacturing, molecular weight controls the dial on dosing systems, measurement tools, and shipment labels. Careers in quality control taught me to trust but verify. Ask three people for the product specs, and don’t move a finger till that formula and weight match across the documentation.
Real talk: lack of a molecular formula or a missing weight slows everything down. Unclear labels mix up shipments. In the classroom, students run the wrong experiments or fail tests from using the wrong calculations. In a compliance job, I watched a batch get quarantined over a formula typo. Last-minute fixes, lost money, and safety hazards all sprang up from that tiny mistake.
Even on a consumer level, hidden details about a product’s chemical backbone can risk health trouble. Food supplements, cleaning products, or even hardware store solvents should clearly show this information. Otherwise, questions about allergies, toxicity, or fires keep getting passed from one person to the next.
Labs and suppliers need to make the formula and molecular weight easy to find. This is not just paperwork—it keeps everyone safe and responsible. Training sessions should cover how to spot formula errors and double-check the math on molecular weights. Digital databases provide quick lookups, yet human review saves time and headaches.
Regulators play a part. Agencies set ground rules for what details must show up on shipping slips, safety sheets, and product packaging. Turning chemical details from fine print to clear facts on every order or bottle helps everyone down the line, from researchers to warehouse staff to end users. People who demand these numbers help raise the standards. They let customers and partners know the company cares, not just about science, but about safety and transparency.
Handling chemicals in the lab can feel like second nature after a while, but all it takes is one slip-up for a routine day to turn sideways. I remember my first lab job—every bottle, no matter how small, got a detailed walk-through from the supervisor. She’d say, chemicals don’t announce themselves when things start to go wrong. They just do. With 1,1,2,2,3,3,4,4,4-Nonafluorobutane-1-Sulphonyl Fluoride (NFBSF), that advice rings true. Even experts need reminders on how best to stow it—and why you don’t want shortcuts in your process.
People working with NFBSF expect a powerful reagent, but they might not realize that it’s keen on reacting with moisture in the air. Water triggers breakdown, releasing hazardous gases. Anyone who’s spent enough time with sulfonyl fluorides knows the smell that leaks from a poorly sealed bottle. For this reason, closed, airtight containers do more than tidy up the bench—they stop dangerous reactions before they start. Those who skimp on simple essentials, like using proper sealing techniques or high-quality bottles, put themselves and colleagues at risk. The right container pays for itself through fewer accidents and more reliable results.
The freezer, or at least a cool, stable spot away from sunlight and heat, always becomes a home for NFBSF bottles. Temperatures up to room temperature—think below 25°C—keep the compound stable. Heat makes it more volatile, unpredictable, and sometimes dangerous. My lab learned this lesson after a single careless placement on a sunny shelf resulted in a leaky, brittle cap—an accident a few degrees cooler could have prevented. Sunlight isn’t just about warmth, either. Ultraviolet rays kick off chemical changes much faster than most imagine. Danger doesn’t always show up in big ways; it creeps in with slow, silent changes in physical properties.
If you’ve stored anything flammable or reactive, you know better than to mix up containers. NFBSF gets along best with acid-proof, hard plastic or glass. Steer clear of metals—the risk of corrosion or dangerous reactions isn’t just theoretical. More than one university has logged reports about improper storage on metal shelving leading to compound instability. Someone new to chemical management often misses that lesson until a professor or senior chemist corrects them. Good habits start with clear instructions and visible labels. Forgetting these steps means someone else could combine incompatible chemicals without knowing it. In my own work, extra care with labels—dates, concentration, and hazard symbols—has saved time and confusion, especially when the next shift rolls around.
Labs shouldn’t rely solely on written protocols. Regular, hands-on training keeps safety fresh in people’s minds. A checklist next to the storage fridge works better than sending out reminders by email. Peer checks have caught more mistakes in real time than once-a-year refresher sessions. Having real case studies—close calls or near misses—at weekly safety meetings reminds everyone why following storage best practices for NFBSF matters. Investing in quality safety infrastructure, like dedicated corrosives cabinets and spill kits close by, gives everyone confidence. It isn’t about fear—just about keeping everyone safe enough to work another day.
As someone who worked behind a counter supplying chemicals to local businesses, I saw the raised eyebrows whenever a customer was handed more than one option for the same product. Some thought it looked like a sales trick—“Regular or premium?” But there’s far more riding on the question of purity or grade than price tags or profit margins.
Take salt, for example. Table salt keeps your dinner tasting just right, but pharmaceutical operations reach for a tin where footnotes explain moisture and impurity levels. If you own a pool, you buy something different yet again. Each use puts a spotlight on purity, and mistakes can have real consequences—clogged equipment, failed reactions, wasted resources, even safety hazards.
Lab and medical workers don’t gamble with purity. Medicines need raw materials that pass strict standards. Many people still recall news about tainted drugs traced to subpar ingredients. A small contaminant—hardly a blip on an industrial scale—lands someone in a hospital bed. Confidence comes from documented, verifiable grades, tracked all the way from producer to user.
Out in the field, factories can’t risk downtime. Engineers pay attention to grades for everything from lubricants to lubricants to cleaning agents. A cheaper alternative can end up snaring an entire system in maintenance delays. It’s easy to scoff at “premium” labels, until a mishap stops the line and ignores all efforts to save costs.
There are more questions about contamination and disposal than ever. Government rules weigh heavily on those who buy or use large volumes of raw materials. A batch with impurities might send heavy metals down the drain, costing more to treat or clean up. Fines stack up quickly for those caught outside compliance. Companies have started to ask their suppliers tougher questions, and stories of fines often spur competitors to follow suit.
I’ve found customers benefit from a frank talk with suppliers about what they need. A restaurant might not pay for pharmaceutical purity, but a research lab expects “trace analysis grade” and gets paperwork to match. Third-party labs keep producers honest; audited testing stops a lot of trouble before it starts. Investing in staff training means fewer mix-ups, less confusion, and more informed decisions on the job.
Gradation and purity aren’t just industry buzzwords or technical lingo. They guide safe handling, reliable results, and responsible environmental practices. Tools such as detailed certificates of analysis, regular supplier reviews, and targeted staff education make a world of difference.
Looking back, I trust suppliers with clear documentation and a habit of answering tough questions. Product grades aren’t cosmetic—they’re the backbone of trust running through food, medicine, industry, and ecosystem management. The questions around purity and grade push everyone—dealers, users, and regulators—to do a better job. As more people grow comfortable asking tough questions, producers feel the heat to be clear, consistent, and honest.
| Names | |
| Preferred IUPAC name | 1,1,2,2,3,3,4,4,4-Nonafluorobutane-1-sulfonyl fluoride |
| Other names |
NfBsa Nonafluorobutanesulfonyl fluoride |
| Pronunciation | /ˌnɒn.ə.flʊˈrɒr.oʊ.bjuːˌteɪn.wʌn.ˈsʌl.fə.nɪl.ˈfluː.əˌraɪd/ |
| Identifiers | |
| CAS Number | 407-25-0 |
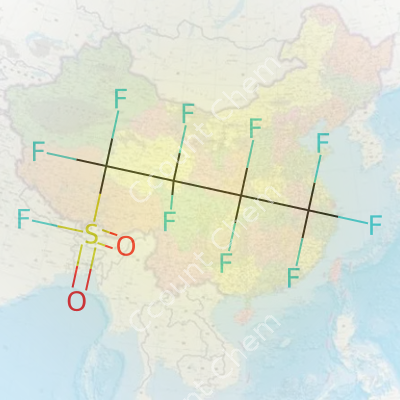
| 3D model (JSmol) | `3D model (JSmol): C(C(C(C(F)(F)S(=O)(=O)F)(F)F)(F)F)(F)F` |
| Beilstein Reference | 3537204 |
| ChEBI | CHEBI:140715 |
| ChEMBL | CHEMBL4295947 |
| ChemSpider | 25505199 |
| DrugBank | DB08760 |
| ECHA InfoCard | 100.196.572 |
| EC Number | 430-67-1 |
| Gmelin Reference | 1451451 |
| KEGG | C19664 |
| MeSH | D000072661 |
| PubChem CID | 10104105 |
| RTECS number | QM4750000 |
| UNII | F536D27P6Z |
| UN number | UN3162 |
| CompTox Dashboard (EPA) | DTXSID80880247 |
| Properties | |
| Chemical formula | C4F9SO2F |
| Molar mass | 324.100 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.7 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.6 |
| Vapor pressure | 2.22E+03 hPa (25 °C) |
| Acidity (pKa) | 5.2 |
| Basicity (pKb) | pKb = 13 |
| Magnetic susceptibility (χ) | -73.6 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.307 |
| Viscosity | 2.2 cP (25°C) |
| Dipole moment | 3.8851 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 354.2 J⋅mol⁻¹⋅K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1682.6 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1682.2 kJ mol-1 |
| Pharmacology | |
| ATC code | D08AK06 |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P262, P280, P304+P340, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | Health: 3, Flammability: 0, Instability: 2, Special: - |
| Flash point | >100 °C (string) |
| Lethal dose or concentration | > Inhalation LC50 Rat (4h): >200 mg/m³ |
| LD50 (median dose) | LD50 (median dose): >2,000 mg/kg (rat, oral) |
| NIOSH | RS6112000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 200 μg/m³ |
| Related compounds | |
| Related compounds |
Trifluoromethanesulfonic acid Nonafluorobutanesulfonic acid 1,1,2,2,3,3,4,4,4-Nonafluorobutanesulfonate Nonafluorobutanesulfonamide |